- ABDA
- ADR
- API
- BAID
- CAP
- CAT
- CESP
- CRO
- CTD
- DOC
- DOCX
- eAF
- eCTD
- EFPIA
- EMA
- EPAR
- ERP
- eSubscription in Europe and US
- EU IDMP Task Force
- EUNDB
- EUTCT
- GINAS
- H+V
- HL7
- HMA
- HTML
- ICD-10
- ICH
- IDMP
- IDMP Cost for the Companies
- IDMP Gap Analysis
- IDMP Stakeholder Mindmap
- IFA
- IMP
- Individual Case Safety Reports (ICSR) in Pharmacovigilance
- ISO
- ISO 11238
- ISO 11239
- ISO 11240
- ISO 11615
- ISO 11616
- ISO TC 215
- Lot Distribution Report LDR
- MAA
- MAH
- MDM
- MedDRA
- NCA
- OMS
- Periodic Adverse Drug Experience Report PADER
- Pharmacovigilance Implementing Measures
- PHARMAZIE.COM
- PhPID
- PMS
- PRAC
- PSMF
- PSUR
- RIM
- RMS
- RTF
- SmPC
- SMS
- SNOMED CT
- SPC
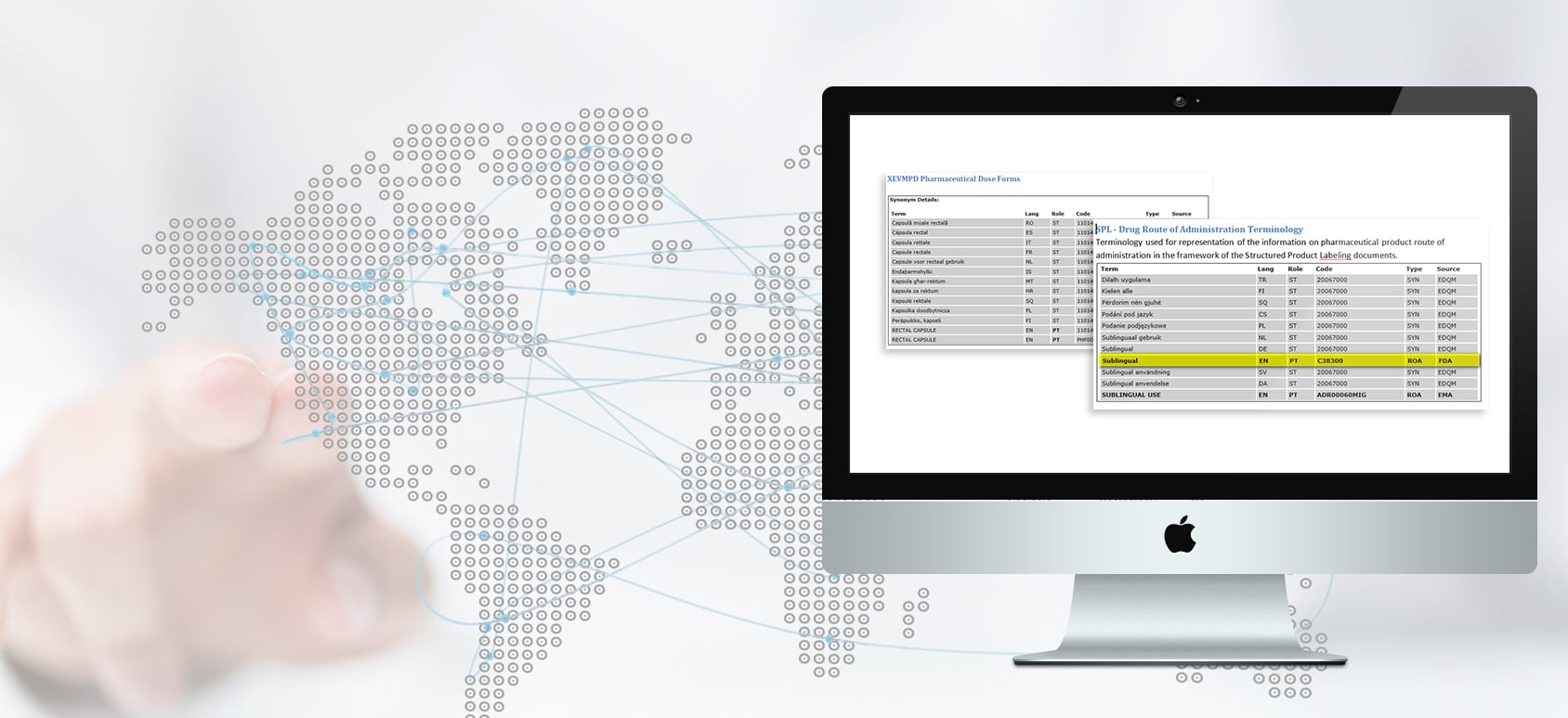
- SPL
- SPOR
- SPOR Data Mapping Activities
- The EU clinical trial portal and database
- UCUM
- XEVMPD
- XML