SPOR
(as of 23.03.2018, Ursula Tschorn, IDMP SPOR Industry Change Liaison)
SPOR is short for Substances Products Organisations and Referentials in the IDMP projects of the EMA. SPOR data services will act as the vehicle for implementation of ISO IDMP standards in the regulatory and the e-health world.
SPOR data services
SPOR is aimed at delivering quality data services on Substances, Products, Organisations and Referentials to power EU regulatory activities.
Four projects have been established
Four projects have been established at the EMA to implement services that centralise management of each of the domains of master data. The four projects are collectively known as SPOR data services and the single once are abbreviated with SMS, PMS, OMS and RMS.
Implementation of the four projects
The implementation of the four projects will be phased.
- All proposals relating to implementation of the projects on Substances, Products, Organisation and Referentials have been and will continue to be consulted on widely with regulators and industry representatives.
- IDMP with its referentials applies to both domains, Human and Veterinary
- In parallel, EMA is implementing the messaging standards developed by Health Level Seven (HL7), which define a format for the electronic exchange of data that is compliant with the ISO IDMP technical specifications.
Use of SPOR in regulatory activities
Adoption of IDMP operating models will facilitate the implementation of consistent, centrally-maintained, ISO IDMP-compliant data, which will feed regulatory activity across the product lifecycle.
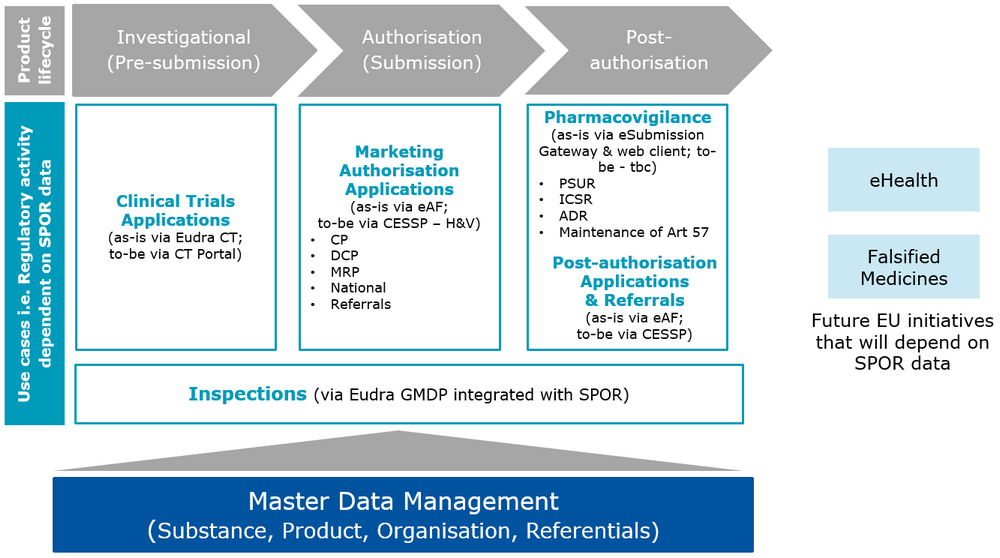
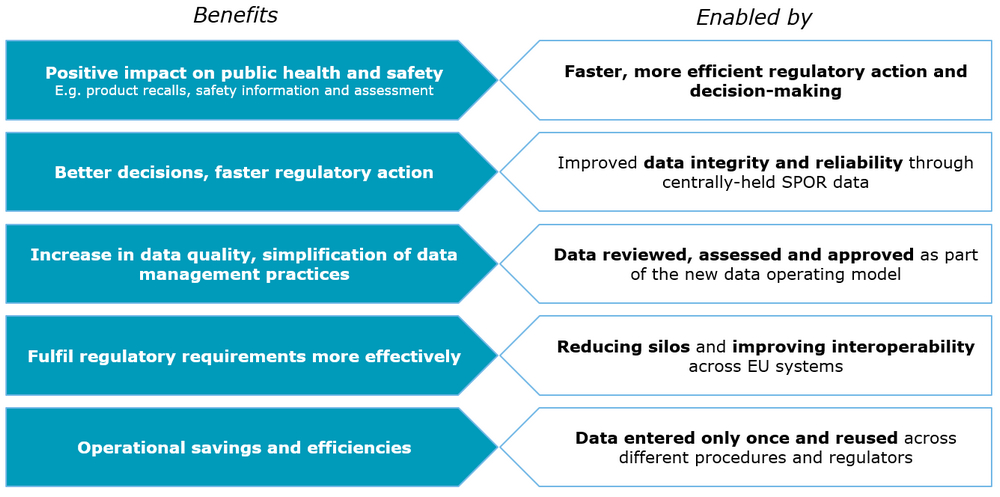
Benefits
Standardised data alone is not sufficient to achieve benefits. The benefits will be realised incrementally:
- As all phases of SPOR are completed; and
- Provided other opportunities for integration are implemented via EU Telematics Programmes such as CESSP, Clinical Trials EU Portal
New ways of accessing SPOR data
There are 4 ways of accessing the SPOR data:
- web interface
- APIs* (Application Programming Interface)
- Draft API specifications have been shared with SPOR Task Force; final API specifications are expected to be published in August
- For RMS, backward compatibility will be maintained with EUTCT for NCAs who use EUTCT
*An API is a mechanism to allow your IT systems to exchange information automatically with RMS and OMS
Download SPOR API Documentation
(Udated 9.1.2018)
A new edition (version 1.14 published 09.01.2018) of the SPOR API documentation (API schemas, OMS & RMS message formats and HL7) is accessible for interested parties for a free download in this website:
The latest version of the API specification incorporates the following additional information:
- A description of how an implementing system must retain rowid elements in the creation, and later management, of change requests in RMS.
- A description on the fields that have to be conditionally provided when dealing with both OMS and RMS change requests depending on the status and the data of such requests.
- An explanation on how OMS retains compatibility for searching elements based on identifiers, even after they have been merged, and how the current identifier, of an element, is highlighted in the response data.
- A description on what kind of change requests can be filed in OMS depending on the status of the referred organisation and/or location.
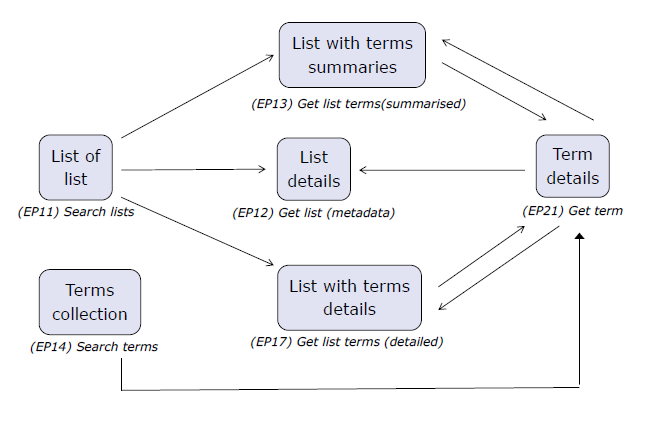
Please note that in the SPOR API specification, the services are set out in Section 6 and those that are OMS-specific are marked “O”, RMS-specific are marked “R”, and services shared by both RMS and OMS are marked as “RO”.
The documents have been shared with the SPOR Task Force and UAT testers.
EMA Data Stewards
EMA will take over the function of a data steward to maintain and coordinate the SPOR data.
- A specialised team of EMA staff that will manage data on behalf of stakeholders and provide user support
- Validate access requests to SPOR services
- Directly involved in maintaining the quality of the data:
Profiling the data (assessing quality of data)
Various data anomalies (different formats of the data e.g. telephone number) can be identified / monitored and data correction can be initiated
Reports generated using this cleansed data will be more reliable - Take action on change requests for new/amended Referential Lists/Terms and Organisation data
Data content
RMS lists at go-live:
- Lists from EUTCT (apart from Substance list)
- Lists to support OMS
- Lists for ISO 11239 (Pharmaceutical dose forms, units of presentation, routes of administration and packaging) and ISO 11240 (Units of measurement)
- Some lists to support PMS project (e.g. Material)
Content of the OMS dictionary at go live:
- MAHs: (H+V) CAPs & (H) NAPs
- MAAs: (H+V) CAPs
- MRL applicants (Vet)
- MA & MRL contacts: (H+V) CAPs
Referentials Management Services RMS Operating Model
[av_image src=’https://www.idmp1.com/wp-content/uploads/2016/09/wiki_spor_rms_process_in_regulatory_context.jpg’ attachment=’8063′ attachment_size=’full’ align=’center’ animation=’no-animation’ link=” target=” styling=” caption=” font_size=” appearance=”][/av_image] [av_button_big label=’Download EMA´s Update on SPOR RMS Process in Regulatory Context (PDF as of 29. November 2016)’ description_pos=’below’ link=’manually,http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/11/WC500217408.pdf’ link_target=’_blank’ icon_select=’yes-left-icon’ icon=’ue82d’ font=’entypo-fontello’ custom_font=’#ffffff’ color=’theme-color’ custom_bg=’#444444′ color_hover=’theme-color-subtle’ custom_bg_hover=’#444444′][/av_button_big]
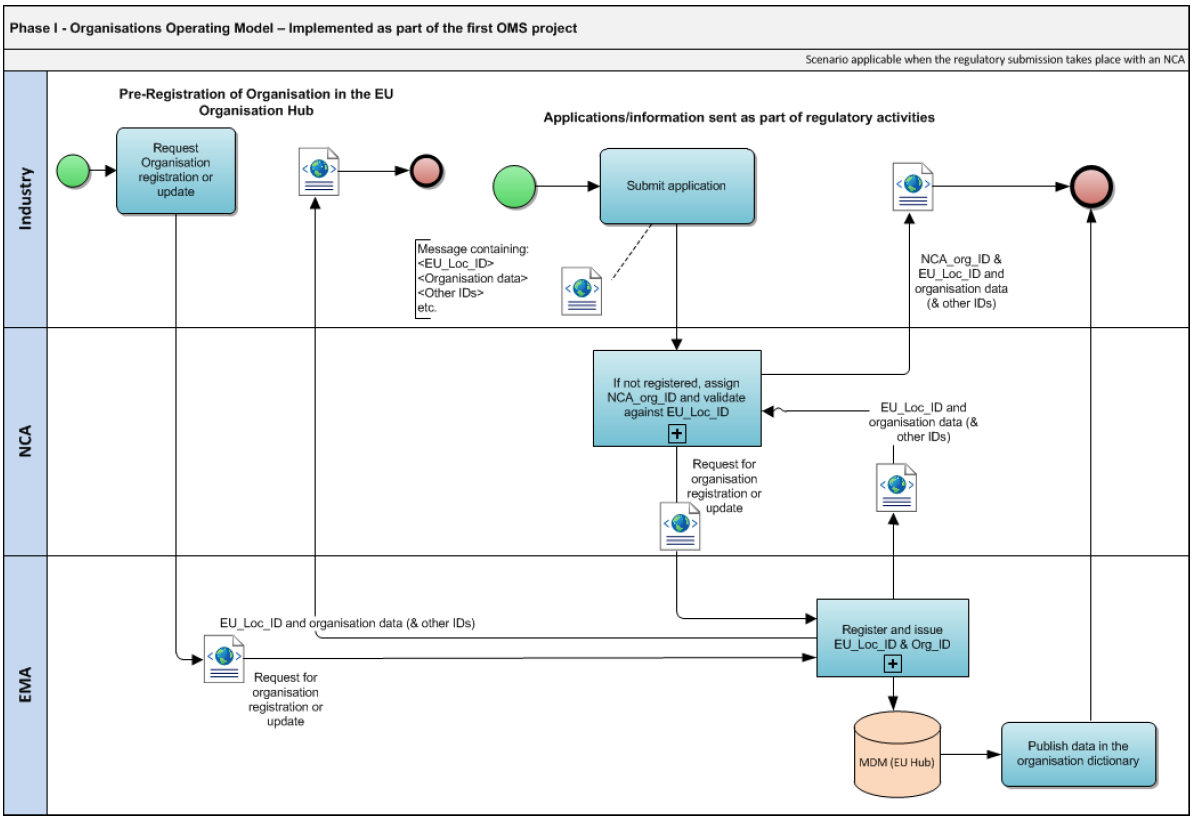
Organisations Management Services OMS Operating Model
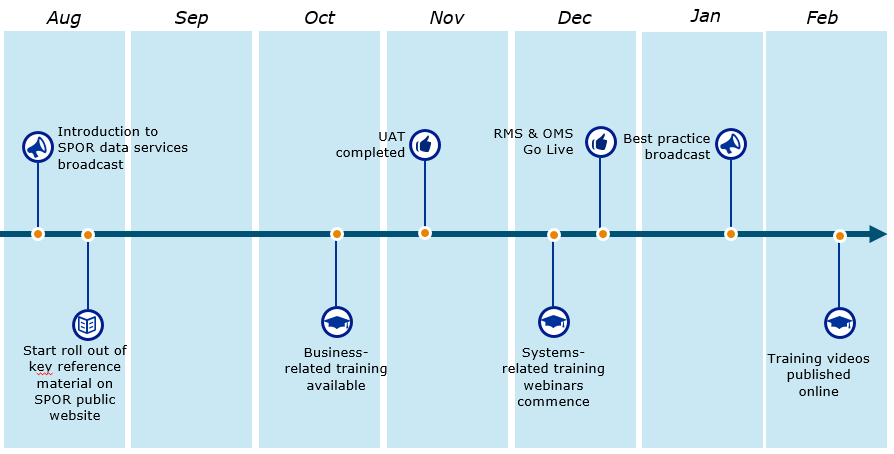
Key Industry engagement activities
- Industry is now asked to start with mapping activities from their local data to IDMP SPOR referentials
- Industry Change Liaisons will provide a continuous channel for information and training throughout the year
- In addition, a number of specific events are planned that will be open to all Industry stakeholders
In summary
- SPOR data services will act as the vehicle for implementation of ISO IDMP standards
- SPOR data services will enable the realisation of benefits at all stages of the product lifecycle due to future integration of regulatory processes with SPOR’s standardised data and central data management services
- Implementation of RMS and OMS is the first step in a phased approach to roll-out of SPOR and of other Programmes dependent on SPOR data
- In order to be ready for the future changes brought about by IDMP and by integration with other Programmes, Industry should prepare now to ensure they have the foundations in place through alignment with RMS and OMS
