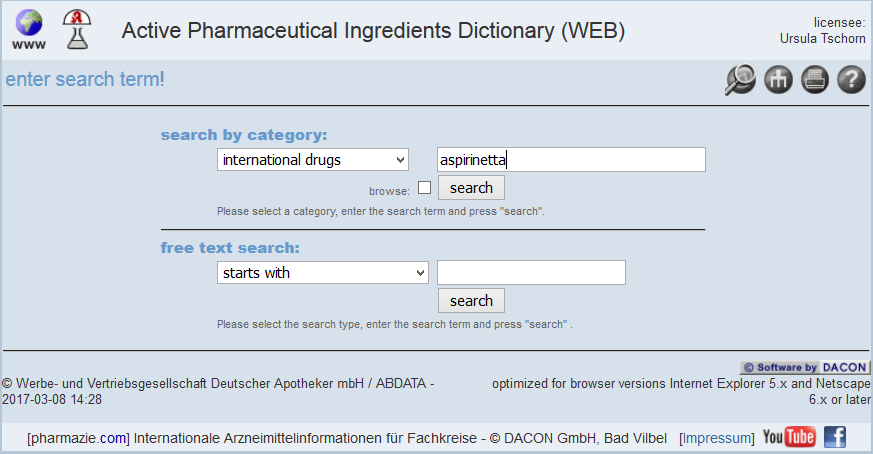
ACTIVE PHARMACEUTICAL INGREDIENTS DICTIONARY WORLDWIDE
ACTIVE PHARMACEUTICAL INGREDIENTS
An active pharmaceutical ingredient (AI) is the ingredient in a pharmaceutical drug that is biologically active
- The term active constituent is often chosen when referring to the active substance in a plant
- The term active substance may be used for natural products
- The inactive ingredients are usually called excipients in pharmaceutical contexts
THE ACTIVE PHARMACEUTICAL INGREDIENTS IN MEDICINAL PRODUCTS
- The Active Pharmaceutical Ingredient (AI) is the part of any drug that is active
- Some medicinal products consist of combination therapies, have multiple active pharmaceutical ingredients to treat different diseases
- All drugs are made up of two core components: the active pharmaceutical ingredient, which is the central ingredient, and the excipient, the substance inside the drug that helps deliver the medication to the patient.
THE ACTIVE PHARMACEUTICAL INGREDIENTS DICTIONARY IN A NUTSHELL
Data for the identification of substances
Chemical/physical properties
Links to further derivatives
Overview of search results
International
Pharmacological and toxicological information
ISO 11238
Regularly updated
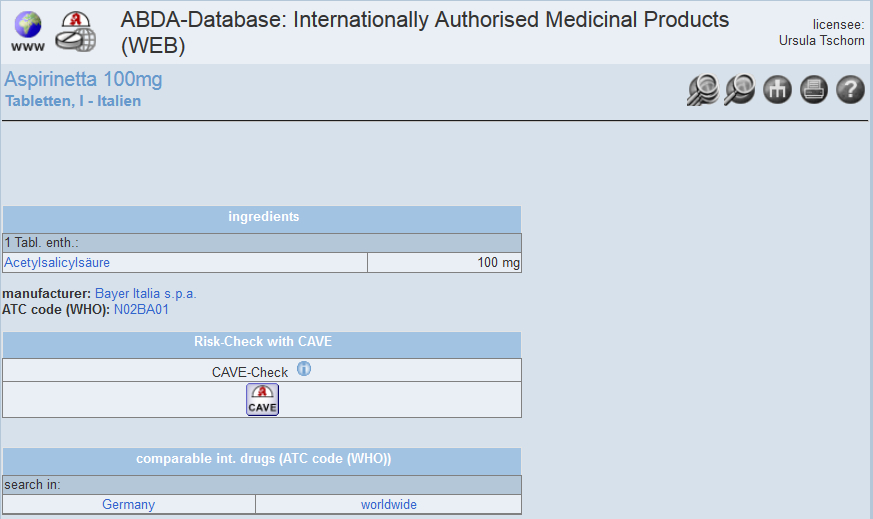
MANUFACTURING OF THE ACTIVE PHARMACEUTICAL INGREDIENT
The manufacturing of the active pharmaceutical ingredient can take place in- or outside the marketing authorisation holder´s home
Quality must be verified by Good Manufacturing Practice (GMP) status and inspection. Even in the case of outsourcing, AIs are subject to stringent regulations and oversight from the country they are shipped to.
"For me as CRO the Active Pharmaceutical Ingredients Dictionary is the best way to find Excipients, active pharmaceutical ingredients of the different drugs used in clinical trials."
President Dinox

