Note: Meet IDMP1 Partner Dieter Schlaps at the RSIDM in North Bethesda Feburary 5-7, 2018
IDMP1 will be present at the upcoming Regulatory Submissions, Information and Document Management Form (RSIDM), at North Bethesda (US), from February 5-7, 2018. If you are there, too, don’t forget to visit us at the booth of our partner IDENTIFICA (booth no. 212).
Despite the announcement, that EMA’s timelines for the introduction and roll-out of IDMP as well as SPOR are postponed beyond the agency’s expected relocation to Amsterdam, we now see a number of instances, where IDMP data are already put in place and are going to be used already in submission activities:
- EMA’s SPOR portal, which currently consists of an organisation database[1] and the referentials database RMS[2] are now available for industry to enter data about their companies and , to review their manufacturers’ data, to verify that their internal systems’ controlled vocabularies are consistent with the referentials available in OMS-RMS, to modify or map data where possible and to request data changes where necessary.
- FDA is in the process of releasing a new CMC guidance, entitled “Pharmaceutical Quality/Chemistry Manufacturing and Controls (PQ/CMC) Data Elements and Terminologies”[1], that will make use of the IDMP standards, especially the identification of manufacturers, and further controlled vocabularies defined in the IDMP standards.
Our Newsletter from August/2017 dealt with that. - EMA’s new Clinical Trials Portal which is going to be rolled out in Q3 of 2018 according to EMA’s “Delivery time frame for the EU portal and EU database” is a likely candidate to make use of SPOR’s OMS data when it comes to Clinical Trial Sponsors. It is also obvious when looking at the kind of controlled vocabularies that are already available in the RMS component of SPOR: A lot of them are dealing with clinical trial information. This is also likely to be reused in the Clinical Trial Portal system.
An architectural sketch of this system that includes EMA’s SPOR solution of this system is shown below (Source: http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2017/03/
pdf). - As described in our blog Falsified Medicines and in EFPIA[3]‘s presentation “EU IDMP Taskforce – Falsified Medicines Directive and European Medicines Verification System Update” from February 2016, the European Medicines Verification System is another solution that will use SPOR-OMS as well as RMS data.
- And finally, the Application Form (eAF) as a Vehicle for IDMP Data Submissions is the first application that is going to make use of the SPOR-OMS right now. In this, Marketing Holder/ Marketing Applicant identification is to be reused from SPOR-OMS. But we expect that other (RMS) data is going to follow later.
Application Form (eAF) as a Vehicle for IDMP Data Submissions Timelines
In the following figure the eAF timeline is shown:
Figure Roadmap for eAF introduction (Source: IRISS eAF Workshop on Jan. 9, 2018)
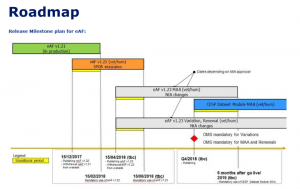 During the transitional periods that are depicted in the figure, only, the applicants are allowed to enter free text data for MAAs or MAHs. But in Q4/2018 the latest, the use of Org-IDs and Loc-IDs for the MAH and MAA organisations and their respective locations will be mandatory.
During the transitional periods that are depicted in the figure, only, the applicants are allowed to enter free text data for MAAs or MAHs. But in Q4/2018 the latest, the use of Org-IDs and Loc-IDs for the MAH and MAA organisations and their respective locations will be mandatory.
For the industry this means, that the marketing authorisation holders must have by then:
- registered in SPOR-OMS,
- defined the internal responsibilities (“IDMP Office”) for managing and maintaining the internal and external organisation data,
- prepared consolidated and up-to-date organisation data
- submitted the consolidated organisation data to SPOR-OMS,
- received the Org-IDs and Loc-IDs for their company and subsidiaries who act as MAHs or MAAs in product submissions
But the use of organisation data from SPOR-OMS is also a paradigm for the future, in such that it shows how structured data will more and more replace freetext data in the electronic forms that are currently used for submissions in Europe, but also in the rest of the world.
Imagine what will happen, when more and more internationally standardized (via IDMP) medicinal product data structures will be used in eAFs:
- A company that has submitted a product in one country using this kind of a future eAF would be able to reuse most of the (e.g. dossier-related) data for another country submission. By this, submissions would become much more efficient, less time would be wasted and a much higher consistency would be achieved.
- Using the relevant IDMP data from the eAF, a Health Authority get in touch with another agency in order to reuse evaluation results or specialized knowhow being available at that other agency in another country. By this, evaluation procedures would become much more efficient as well as consistent between the agencies.
- Using eAFs that are able to handle embedded IDMP data, countries that cannot afford or do not (yet) intend to implement fully-fledged IDMP portals, the eAF would provide an efficient possibility (and relatively low-cost alternative) to “jump on the IDMP bandwagon” and to do “cherry-picking” by reusing IDMP data that was submitted elsewhere. By this, they still would be able to benefit from high-quality, standardized IDMP data.
Conclusion and Outlook to the Application Form (eAF) as a Vehicle for IDMP Data Submissions
Both EMA as well as FDA obviously have chosen a pragmatic approach to introduce the concepts, identifiers and systems from the IDMP standards.
The eAF with embedded SPOR-OMS data is seen as a paradigm for further structured data to be included in electronic application forms. There are significant benefits expected from this which all result from the opportunity to reuse the structured data for a number of use cases.
This will bring significant benefits for the pharmaceutical company, the health authority, and, last, but not least, the patient.
Link to EMA´s Publication giving more Details on the Application Form (eAF) as a Vehicle for IDMP Data Submissions
EMA´s SPOR Webinar on Using OMS and RMS in eAF (as of 28.11.2017)
References:
[1] Pharmaceutical Quality/ Chemistry Manufacturing and Controls (PQ/CMC) Data Elements and Terminologies, círculated by the FDA in August 2017 for annotation and reviewNote:
IDMP1 will be present at the upcoming Regulatory Submissions, Information and Document Management Form (RSIDM), at North Bethesda, from February 5-7, 2018. If you are there, too, don’t forget to visit us at the booth (no. 212) of our partner IDENTIFICA.
[1] SPOR-OMS: Organisation management system component
[2] SPOR – RMS: Referentials management system component
[3] European Federation of Pharmaceutical Industries and Associations


