ISO 11615 establishes definitions and concepts required for the detailed description and unique identification of medicinal products.
The scope of ISO 11615
ISO 11615 describes data elements and regulated medicinal product information during their entire life cycle (development, authorisation, post-marketing and renewal or withdrawal from the market) and is composed of several ISO Standards.
Taken together all ISO IDMP standards (EN ISO 11615/DTS 20443, EN ISO 11616/DTS 20451, EN ISO 11238/DTS 19844, EN ISO 11239/DTS 20440 and EN ISO 11240) define, characterize and uniquely identify regulated medicinal products for human use from approval, to post-marketing and renewal or withdrawal from the market, where applicable.
Furthermore, to support successful information exchange in relation to the unique identification and characterization of medicinal products, the use of HL7 Common Product Model and Structured Product Labeling (SPL) messaging aspects is also described.
In addition, reference to the use of other normative IDMP and messaging standards for Medicinal Product information is included in this Technical Specification in order to support successful related information exchange.
Information for an authorized medicinal product
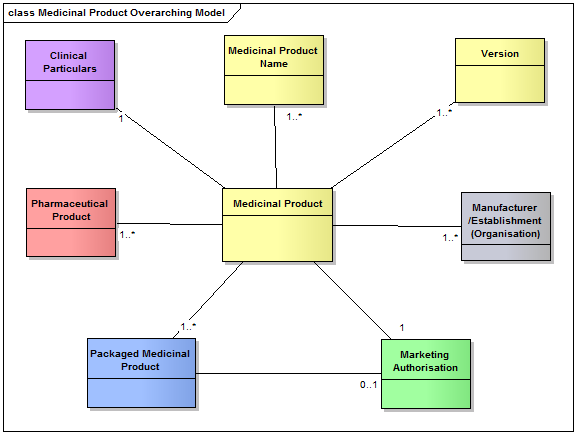
ISO 11615 describes the main concepts modelled to identify a medicinal product.
ISO Standards included in ISO 11615
The following normative standards are essential components for successful implementation of the exchange of IDMP data elements:
- EN ISO 11615:2012, Health Informatics -Identification of medicinal products – Data elements and structures for the unique identification and exchange of regulated medicinal product information
- EN ISO11616:2012, Health informatics – Identification of Medicinal Products – Data elements and structures for the unique identification and exchange of regulated pharmaceutical product information
- EN ISO 11238:2012, Health informatics — Identification of Medicinal Products — Data elements and structures for the unique identification and exchange of regulated information on substances
- EN ISO 11239:2012, Health Informatics — Identification of Medicinal Products — Data elements and structures for the unique identification and exchange of regulated information on pharmaceutical dose forms, units of presentation, routes of administration and packaging
- EN ISO 11240:2012, Health informatics — Identification of Medicinal Products — Data elements and structures for the unique identification and exchange of units of measurement