ISO 11616 International Standard “Health informatics — Identification of Medicinal Products — Data elements and structures for the unique identification and exchange of regulated pharmaceutical product information”.
ISO 11616 is intended to assist pharmaceutical companies, medicines regulatory authorities and all other interested parties in implementing systems and constructing transmittable messages pertaining to pharmaceutical product identification.
Pharmaceutical Product Identification in ISO 11616
The following principles for the unique identification of a pharmaceutical product shall apply in different strata and levels
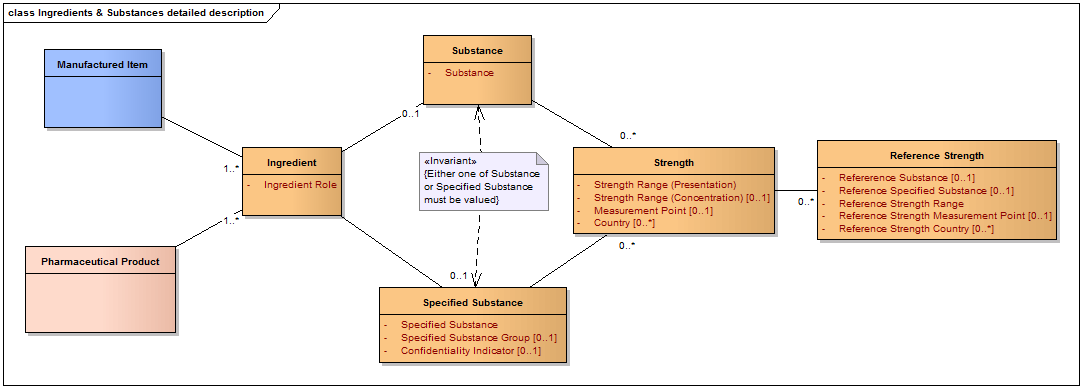
Pharmaceutical Product Identification (PhPID) shall be based on the following subset of elements that describe the pharmaceutical product (See also Figure above):
- active substance(s)/specified substance(s);
- strength(s), strength units (units of measurement and/or unit of presentation);
- reference strengths;
- administrable dose form;
- medical device, when it is a component of a medicinal product.
- adjuvant, when it is a component of a pharmaceutical product
Representation of pharmaceutical products in IDMP
Pharmaceutical identifiers and elements shall represent pharmaceutical products as represented in a medicinal product per the authorization by a regulatory authority.
PhPID Active Substance Identification:
- PhPID_SUB_L1 → Substance(s) Term
- PhPID_ SUB _L2 → Substance Term(s) + Strength + Reference Strength
- PhPID_ SUB _L3 → Substance Term(s) + Administrable Dose Form
- PhPID_ SUB _L4 → Substance(s) Term+ Strength + Reference Strength + Administrable Dose Form