What is a Clinical Trial?
A Clinical Trial is a study performed to investigate the safety or efficacy of a medicine. For human medicines, these studies are carried out in human volunteers.
The Clinical Trial Portal and IDMP SPORS
EMA’s new Clinical Trials Portal is going to be rolled out in Q3 of 2018 according to EMA’s “Delivery time frame for the EU portal and EU database”(under revision). Such as in the Falsified Medicines and the Electronic Application Form (eAF), the Clinical Trial Portal is a likely candidate to make use of SPOR’s OMS data when it comes to Clinical Trial Sponsors.
It is obvious when looking at the kind of controlled vocabularies that are already available in the RMS component of SPOR: A lot of them are dealing with clinical trial information. This is also likely to be reused in the Clinical Trial Portal system.
What is the Clinical Trial Portal and Database aimed at?
- Single EU entry point for clinical trial applications
- Enables supervision at EU level, including inspections
- Provides workspace collaboration tools, workflow and document management capabilities
- Provides publicly available information
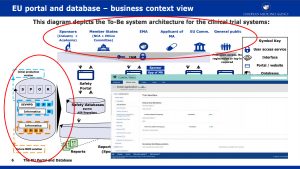
An architectural sketch of this system that includes EMA’s SPOR solution of this system is shown below (Source: EMA).
Public website
Through the website, members of the public can access detailed information on all clinical trials conducted in the EU, in all official EU languages. The website will provide the following features:
- overview of clinical trial statistics;
- advanced search;
- download data and reports;
- site updates and announcements.
Implementation
Although the Regulation was adopted and entered into force in 2014, the timing of its application depends on confirmation of full functionality of the EU portal and database through an independent audit. The Regulation becomes applicable six months after the European Commission publishes notice of this confirmation.
EMA’s Management Board endorsed a delivery timeframe in December 2015. However, due to technical difficulties with the development of the IT systems, the portal’s go-live date was postponed.
Update: The development is progressing, though still requires close monitoring. More precision of the delivery timeframe will be possible after a planned cycle of extensive testing by Member States and sponsor representatives and when further progress with the auditable version of the system has been made.
The audit will be carried out in 2018. EMA will provide further information on timelines after the audit.
The development remains aligned to the schedule that enables the EU Clinical TrialRegulation to come into application in the second half of 2019.

