IDMP Standards
IS SHORT FOR ‘IDENTIFICATION OF MEDICINAL PRODUCTS’
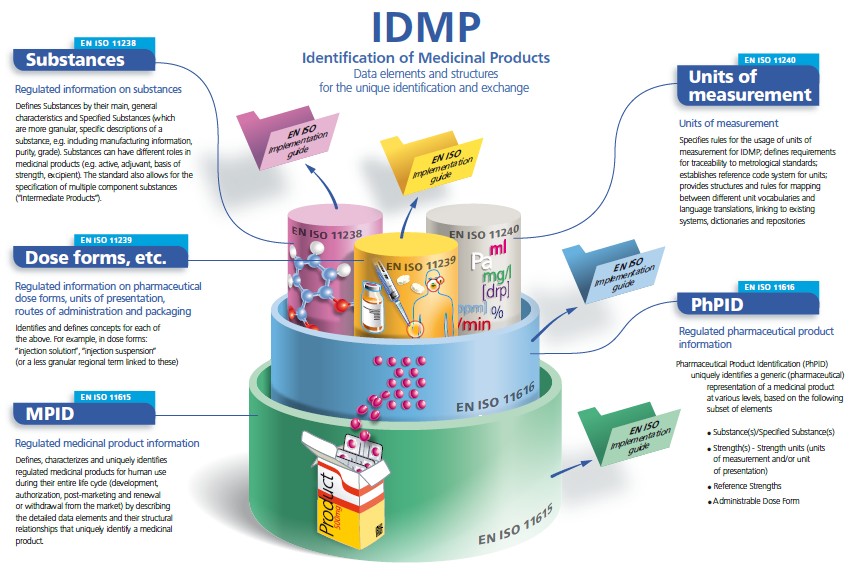
IDMP in a nutshell
The IDMP Standards are a set of 5 ISO international standards that has been developed in response to a worldwide demand for internationally harmonised specifications for identification and description of medicinal products. IDMP provides the basis for the unique identification of medicinal products, which facilitates the activities of medicines regulatory agencies worldwide by jurisdiction for a variety of regulatory activities (development, registration and life cycle management of medicinal products, pharmacovigilance, and risk management). They can also be applied to Investigational Medicinal Products (IMP).
In IDMP Standards messaging specifications are included as an integral part. They describe and protect the integrity of the interactions for the submission of regulated medicinal product information in the context of the unique product identification; they include acknowledgement of receipt including the validation of transmitted information. Health Level Seven (HL7) Message Exchange are normative within the IDMP Standards.
IDMP Standards are completed with Implementation Guides which are currently in development (2015), as well as with Technical Specifications (TS) 16791 (provides guidance for the identification of medicinal products by using intenational supply chain Standards, securing traceability, safe supply chain and other market requirements) and Technical Requirements (TR) 14872 (Requirements for the implementation of the Standards for the identification of medicinal products for the exchange of regulated medicinal product Information), the latter being in development.
