IDMP Drug Dictionary
MORE THAN 120.000 INTERNATIONAL MARKETED MEDICINAL PRODUCTS
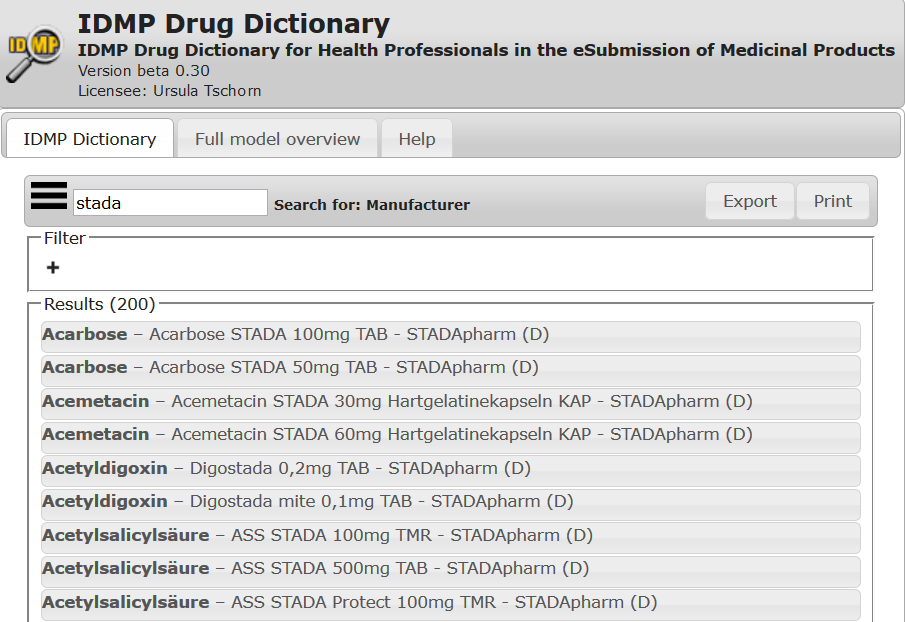
The IDMP Drug Dictionary offers information on medicinal products worldwide structured conform to IDMP requirements.
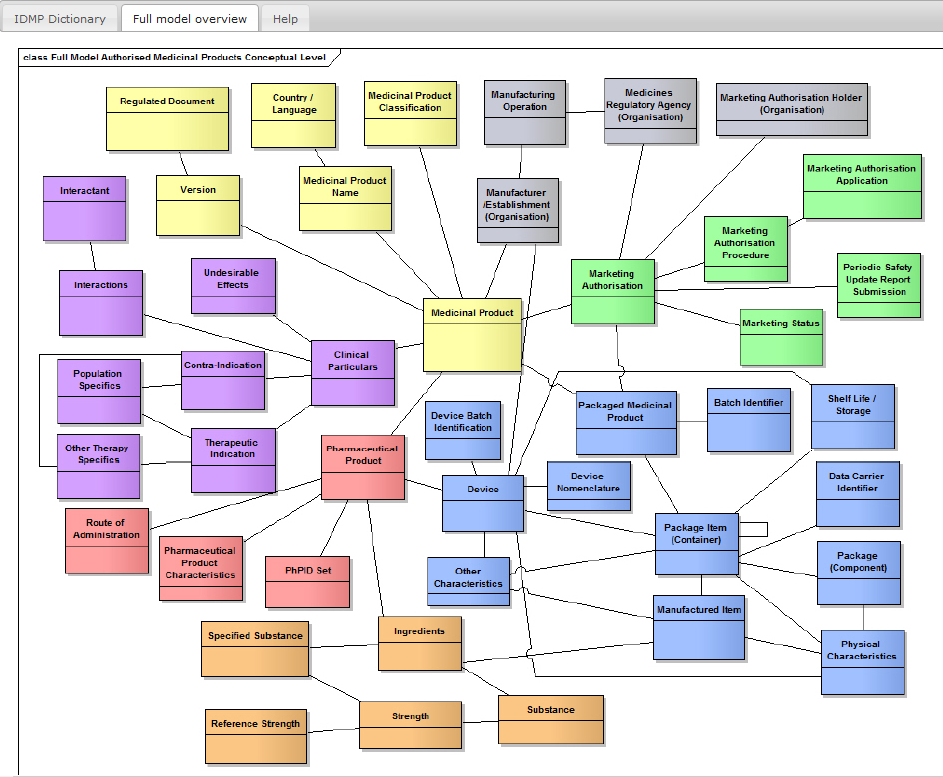
STRUCTURE IS COMPLIANT TO ISO IDMP 11615 AND ISO IDMP 11616
Substances with link to the Global Ingredients Archive System (GINAS), Clinical Particulars structured in Indications with Co-Morbidity and Intended Effect , Side Effect with Frequencies, Interactions, Contraindications.
ALL CORE FEATURES OF THE IDMP DRUG DICTIONARY
Structured data to your medicinal products
Conform to ISO IDMP 11615 and ISO IDMP 11616
International
Including Clinical Particulars
Regularly updated
"For me as CRO the IDMP Drug Dictionary is a revolutionary tool to increase adverse event specification to enhance the outcome quality of clinical trials."
President Dinox